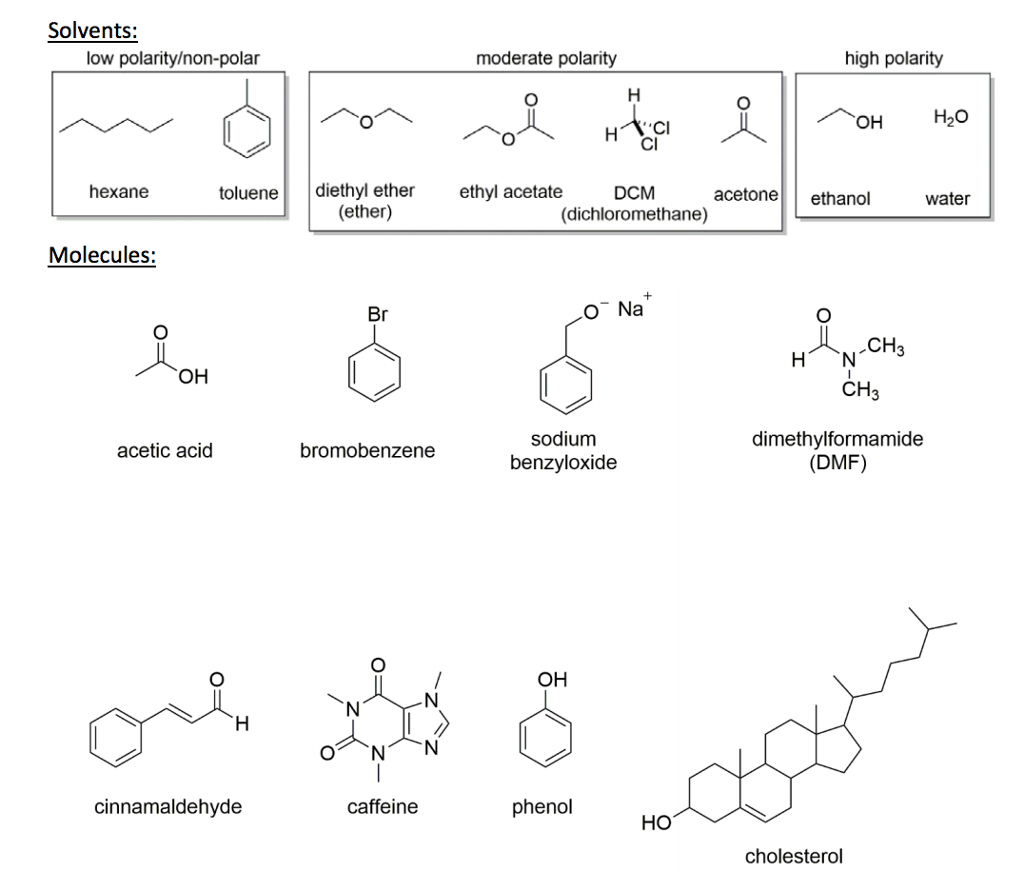
Why Is Acetone More Polar Than Ethyl Acetate . However, removing the solvent completely on a rotary. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine. what is the order of the following compounds from more polar to less polar? Commonly used in 0 to 30% concentrations. 1) ethyl acetate/hexane: ethyl acetate more polar than hexane, but not so much that it is miscible with water. common solvents arranged from the least polar to the most polar. significantly less polar than ethyl acetate. As the percentage (or ratio) of ethyl acetate in the mixture increases, the polarity of the. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic,. Hydrochloric acid, methanol, hexane, petroleum benzene. It is a polar organic solvent.
from ask.modifiyegaraj.com
Hydrochloric acid, methanol, hexane, petroleum benzene. It is a polar organic solvent. As the percentage (or ratio) of ethyl acetate in the mixture increases, the polarity of the. significantly less polar than ethyl acetate. common solvents arranged from the least polar to the most polar. However, removing the solvent completely on a rotary. 1) ethyl acetate/hexane: Commonly used in 0 to 30% concentrations. ethyl acetate more polar than hexane, but not so much that it is miscible with water. what is the order of the following compounds from more polar to less polar?
Ethyl Acetate Polar Or Nonpolar Asking List
Why Is Acetone More Polar Than Ethyl Acetate Hydrochloric acid, methanol, hexane, petroleum benzene. ethyl acetate more polar than hexane, but not so much that it is miscible with water. Commonly used in 0 to 30% concentrations. common solvents arranged from the least polar to the most polar. However, removing the solvent completely on a rotary. what is the order of the following compounds from more polar to less polar? As the percentage (or ratio) of ethyl acetate in the mixture increases, the polarity of the. 1) ethyl acetate/hexane: It is a polar organic solvent. Hydrochloric acid, methanol, hexane, petroleum benzene. significantly less polar than ethyl acetate. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic,. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine.
From www.nagwa.com
Question Video Determining Whether Some Common Simple Molecular Why Is Acetone More Polar Than Ethyl Acetate ethyl acetate more polar than hexane, but not so much that it is miscible with water. common solvents arranged from the least polar to the most polar. 1) ethyl acetate/hexane: However, removing the solvent completely on a rotary. what is the order of the following compounds from more polar to less polar? It is a polar. Why Is Acetone More Polar Than Ethyl Acetate.
From exoncvmev.blob.core.windows.net
Solvents For Tlc at Janice Timmons blog Why Is Acetone More Polar Than Ethyl Acetate Commonly used in 0 to 30% concentrations. 1) ethyl acetate/hexane: common solvents arranged from the least polar to the most polar. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine. As the percentage (or ratio) of ethyl acetate in the mixture increases, the polarity of the. significantly less polar than ethyl acetate. It is a polar organic. Why Is Acetone More Polar Than Ethyl Acetate.
From www.chemistrysteps.com
Solubility of Organic Compounds Chemistry Steps Why Is Acetone More Polar Than Ethyl Acetate what is the order of the following compounds from more polar to less polar? Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine. ethyl acetate more polar than hexane, but not so much that it is miscible with water. Commonly used in 0 to 30% concentrations. 1) ethyl acetate/hexane: However, removing the solvent completely on a rotary.. Why Is Acetone More Polar Than Ethyl Acetate.
From www.coursehero.com
[Solved] answer all the questions Part III. Polarity (Bond polarity Why Is Acetone More Polar Than Ethyl Acetate 1) ethyl acetate/hexane: However, removing the solvent completely on a rotary. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic,. As the percentage (or ratio) of ethyl acetate in the mixture increases, the polarity of the. common solvents arranged from the least polar to the most polar.. Why Is Acetone More Polar Than Ethyl Acetate.
From www.istockphoto.com
Ethyl Acetate Ethyl Ethanoate Molecule It Is Acetate Ester Polar Why Is Acetone More Polar Than Ethyl Acetate 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic,. ethyl acetate more polar than hexane, but not so much that it is miscible with water. significantly less polar than ethyl acetate. 1) ethyl acetate/hexane: However, removing the solvent completely on a rotary. Commonly used in 0. Why Is Acetone More Polar Than Ethyl Acetate.
From www.numerade.com
SOLVED Explain the polarity of acetone, ethanol and ethyl acetate Why Is Acetone More Polar Than Ethyl Acetate As the percentage (or ratio) of ethyl acetate in the mixture increases, the polarity of the. It is a polar organic solvent. Hydrochloric acid, methanol, hexane, petroleum benzene. common solvents arranged from the least polar to the most polar. 1) ethyl acetate/hexane: Commonly used in 0 to 30% concentrations. 23 rows however, acetone is still considered a. Why Is Acetone More Polar Than Ethyl Acetate.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Why Is Acetone More Polar Than Ethyl Acetate 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic,. Hydrochloric acid, methanol, hexane, petroleum benzene. common solvents arranged from the least polar to the most polar. Commonly used in 0 to 30% concentrations. As the percentage (or ratio) of ethyl acetate in the mixture increases, the polarity of. Why Is Acetone More Polar Than Ethyl Acetate.
From ask.modifiyegaraj.com
Ethyl Acetate Polar Or Nonpolar Asking List Why Is Acetone More Polar Than Ethyl Acetate As the percentage (or ratio) of ethyl acetate in the mixture increases, the polarity of the. 1) ethyl acetate/hexane: It is a polar organic solvent. common solvents arranged from the least polar to the most polar. However, removing the solvent completely on a rotary. significantly less polar than ethyl acetate. 23 rows however, acetone is still. Why Is Acetone More Polar Than Ethyl Acetate.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Why Is Acetone More Polar Than Ethyl Acetate 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic,. It is a polar organic solvent. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine. Commonly used in 0 to 30% concentrations. However, removing the solvent completely on a rotary. what is the order of the following compounds from. Why Is Acetone More Polar Than Ethyl Acetate.
From www.researchgate.net
Why we use ACETONE in "acetone insolubility" study instead of other Why Is Acetone More Polar Than Ethyl Acetate 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic,. However, removing the solvent completely on a rotary. It is a polar organic solvent. As the percentage (or ratio) of ethyl acetate in the mixture increases, the polarity of the. significantly less polar than ethyl acetate. common solvents. Why Is Acetone More Polar Than Ethyl Acetate.
From www.chemicalbook.com
Is acetonitrile a highly polar solvent?_Chemicalbook Why Is Acetone More Polar Than Ethyl Acetate Hydrochloric acid, methanol, hexane, petroleum benzene. It is a polar organic solvent. common solvents arranged from the least polar to the most polar. As the percentage (or ratio) of ethyl acetate in the mixture increases, the polarity of the. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine. Commonly used in 0 to 30% concentrations. 1) ethyl acetate/hexane:. Why Is Acetone More Polar Than Ethyl Acetate.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Why Is Acetone More Polar Than Ethyl Acetate Hydrochloric acid, methanol, hexane, petroleum benzene. It is a polar organic solvent. 1) ethyl acetate/hexane: As the percentage (or ratio) of ethyl acetate in the mixture increases, the polarity of the. what is the order of the following compounds from more polar to less polar? However, removing the solvent completely on a rotary. ethyl acetate more polar. Why Is Acetone More Polar Than Ethyl Acetate.
From www.numerade.com
SOLVED Rank hexane, water, ethanol, acetone, and ethyl acetate from Why Is Acetone More Polar Than Ethyl Acetate It is a polar organic solvent. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine. Commonly used in 0 to 30% concentrations. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic,. significantly less polar than ethyl acetate. ethyl acetate more polar than hexane, but not so much. Why Is Acetone More Polar Than Ethyl Acetate.
From ar.inspiredpencil.com
Ethyl Acetate Polarity Why Is Acetone More Polar Than Ethyl Acetate ethyl acetate more polar than hexane, but not so much that it is miscible with water. significantly less polar than ethyl acetate. Commonly used in 0 to 30% concentrations. As the percentage (or ratio) of ethyl acetate in the mixture increases, the polarity of the. common solvents arranged from the least polar to the most polar. However,. Why Is Acetone More Polar Than Ethyl Acetate.
From ar.inspiredpencil.com
Ethyl Acetate Polarity Why Is Acetone More Polar Than Ethyl Acetate ethyl acetate more polar than hexane, but not so much that it is miscible with water. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic,. Hydrochloric acid, methanol, hexane, petroleum benzene. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine. As the percentage (or ratio) of ethyl acetate. Why Is Acetone More Polar Than Ethyl Acetate.
From www.numerade.com
SOLVED Which among the following solvents is a) least polar b) most Why Is Acetone More Polar Than Ethyl Acetate significantly less polar than ethyl acetate. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic,. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine. common solvents arranged from the least polar to the most polar. However, removing the solvent completely on a rotary. Commonly used in 0. Why Is Acetone More Polar Than Ethyl Acetate.
From studylib.net
Acetone as an Alternative to Ethyl Acetate Why Is Acetone More Polar Than Ethyl Acetate It is a polar organic solvent. common solvents arranged from the least polar to the most polar. what is the order of the following compounds from more polar to less polar? However, removing the solvent completely on a rotary. As the percentage (or ratio) of ethyl acetate in the mixture increases, the polarity of the. 23 rows. Why Is Acetone More Polar Than Ethyl Acetate.
From www.numerade.com
SOLVED 3 The structural formulas, and boiling points, for three Why Is Acetone More Polar Than Ethyl Acetate However, removing the solvent completely on a rotary. ethyl acetate more polar than hexane, but not so much that it is miscible with water. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine. Hydrochloric acid, methanol, hexane, petroleum benzene. common solvents arranged from the least polar to the most polar. Commonly used in 0 to 30% concentrations. As. Why Is Acetone More Polar Than Ethyl Acetate.